
 Fazlutdinov K.K.
Fazlutdinov K.K.
 03.04.2020 (updated 11.05.2021)
03.04.2020 (updated 11.05.2021)
 15863 views
15863 views
MDO | Microarc oxidation. The mechanism and technology of applying an oxide coating on aluminum.
Content:
1. General information about microarc oxidation of aluminum (MAO).
2. The composition of electrolytes for MAO.
3. Structure and composition of the oxide coating during MAO.
4. Properties of micro-arc oxide coating on aluminum.
1. General information about microarc oxidation of aluminum (MAO).
Aluminum is one of the most sought-after construction materials today. It is distinguished at the same time by its lightness, strength, electrical and thermal conductivity, corrosion resistance. Under the influence of atmospheric oxygen or other oxidizing agents, aluminum is easily passivated - a natural oxide film (Al2O3) with a thickness of 0.002-0.005 microns appears on its surface. The passive film significantly inhibits corrosion processes on aluminum in atmospheric conditions, despite the fact that it is a very active metal in itself.
Al2O3 is stable in neutral and slightly acidic solutions, has pronounced dielectric properties and is one of the hardest compounds in nature. The disadvantage of the natural protection of aluminum is the small thickness of the passive film. For this reason, it does not provide it with adequate resistance to either corrosion in aggressive environments or abrasion.
To improve these parameters, the thickness of the oxide film must be artificially increased. This process is called oxidation.
Oxidation of metals, in principle, is carried out by thermal, chemical, anodic and microarc methods.
For aluminum, the last three oxidation methods are used:
- Chemical oxidation significantly loses to anode and microarc in terms of performance, but is the simplest and cheapest.
- Good results can be obtained with anodic oxidation (the most common method).
- The best coatings are obtained with MAO, but this is also the most expensive, complex and energy-intensive process.
MAD is a relatively new method of modifying the surface of aluminum. It was developed at the Institute of Inorganic Chemistry of the Siberian Branch of the Russian Academy of Sciences in 1969 under the direction of G.A. Markov. MAO makes it possible to apply heavy-duty oxide coatings with unique protective, electrically insulating, and decorative properties. In appearance, the coating obtained by the microarc method is very similar to ceramics. The process is applicable not only to aluminum, but also to other valve group metals such as Ti, Zr, Mg, Ta, Be.
MAO is performed in an electrolyte solution under current, just like anodizing, but differs from it by using a much higher voltage and a high density electric current. When such a current passes through the metal-electrolyte interface, chaotic microplasma discharges with high temperatures appear on the surface of the part, which outwardly looks like a luminous halo. These microdischarges have a plasma-chemical and thermal effect on the coating and electrolyte. At the discharge site, a film is formed from oxidized forms of the base metal and electrolyte components. It is possible to obtain coatings with different thickness, porosity and properties by selecting the desired oxidation mode and electrolyte composition.
2. The composition of electrolytes for MAO.
The composition of the electrolyte in MAO, along with the substrate material, processing mode and time, is the determining factor of the process.
Electrolytes are used for MAO:
- not having components that form insoluble oxides: solutions of sulfuric, phosphoric acid, alkali. The coatings formed in such electrolytes deepen into the metal due to its oxidation.
- which contain cations or anions that form insoluble oxides and hydrolysis products: aluminate and silicate-alkaline solutions, as well as solutions containing soluble phosphates, bicarbonates and molybdates). After thermolysis, these electrolyte components in the discharge zone become part of the coating and give an additional increase in the size of the part after the formation of the oxide layer.
The applied MDO modes differ by:
- type of current (DC, AC, AC superimposed on DC);
- applied voltage polarity;
- changing electrical parameters (galvanostatic, galvanodynamic, potentiostatic, potentiodynamic, constant or falling power modes);
- the nature of the discharge (spark, microarc, arc, arc electrophoresis);
- degrees of control (manual, semi-automatic, automatic).
Bath voltage is 600-1000 V, current density is up to 30 A/dm2, specific power consumption reaches 11000-30000 W/dm< sup>2. For comparison, when anodizing, the output voltage is in the range of 12-180 V (large values are used extremely rarely), the current density is 0.5-2 A/dm2, the specific power consumption is only 6-360 W/ dm2. Chemical oxidation is carried out without current at all.
No special surface preparation required prior to coating.
In practice, the process of microarc oxidation is carried out mainly in weakly alkaline electrolytes when pulsed or alternating current is applied.
3. Structure and composition of the oxide coating during MAO.
Anode microarc discharges pass between the surface of the oxide film and the electrolyte, heating the film to high temperatures of 1000-2000°C. At such temperatures, thermal destruction of water occurs with the formation of atomic and ionized oxygen. High-temperature phases are formed in the coating (corundum .alpha.-Al2O3), decomposition of the electrolyte components and their interaction with base metal oxides occur. Thus, the MAO coating is not purely oxide, but has a complex composition and structure.
The resulting oxide layer is formed approximately 70% deep into the base metal and only 30% of the coating goes beyond the original dimensions of the part.
The metal-oxide-discharge-electrolyte system implemented in MAO has ionic conductivity, the current flows through the discharge channels. Therefore, the formation of pores in the coating is a prerequisite for its formation.
MAO coating has a layered structure, an example of which is shown in Figure 1:
- The outer layer (technological) is loose. When using an alkaline electrolyte with the addition of liquid glass, this layer consists of mullite Al2O3*2SiO2
- The inner layer is dense, having a high microhardness. Composed of aluminum oxide Al2O3.
- The transition layer is thin, from 0.01 - 0.1 microns, located between the substrate material and the oxide layer.
The top loose layer is often removed by sandblasting and the part with a dense oxide coating comes into service.
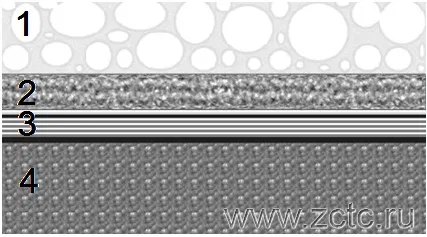
Figure 1 — Layered structure of the coating obtained by the MAO method: 1 - outer (technological) layer, 2 - dense (working) layer: 3 - transition layer: 4 - base material.
Coating composition depends on processing time. An example is shown in Figure 2.
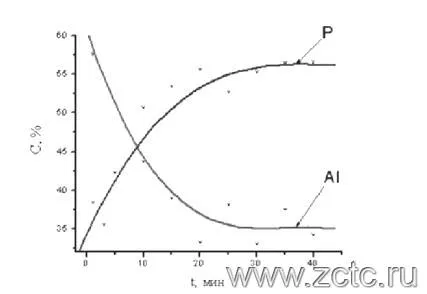
Figure 2 — Change in the content of aluminum and phosphorus on the surface of the MAO coating from the time of treatment in a phosphate electrolyte.
4. Properties of micro-arc oxide coating on aluminum.
- MAO coating is a layer of ceramic up to 300-400 microns thick, which, along with the composition, determines its properties.
- From the point of view of appearance after MAO, coatings of brown, black, brown, blue and white can be obtained. For example, on alloy D16 a black or brown coating is obtained, on B95 - pink, AMg - beige, AK12 - gray. Alloys with titanium after coating have a blue tint. An admixture of color is given by compounds of alloying components.
- The microhardness of the coating can reach 2500 kg/mm2. In terms of wear resistance, this coating is not inferior to tungsten carbide.
- MAO coating is chemically resistant to acids and alkalis, not to mention atmospheric conditions.
- The average breakdown voltage of the coating is 600 V. With additional filling of the pores, the breakdown voltage value rises to 2500 V. The breakdown voltage of the coatings, their hardness and resistance to corrosion depend on the thickness of the coating, the type and size of the pores.
- The porosity of the coatings varies in the range of 5-50%, the pore sizes are from 0.01 - 10 microns.
The structure of pores with a coating thickness of more than 5-10 microns is complex, with various branches and an abundance of closed spaces. If necessary, porosity can be reduced by impregnating with polymers (PTFE), dyes or oil up to 2-3%.
The thickness of the MAO coating is selected based on its purpose and operating conditions. For example:
- 5-10 microns are enough for applying a sublayer for staining,
- Decorative and anticorrosive properties in atmospheric conditions provide 20-40 microns of coating.
- 50-100 microns or more are needed to impart electrical insulating properties or high wear resistance.
Increasing the content of alloying elements in the metal causes a reduction in the thickness of the coating by 2 - 7.5%, compared to the thickness that could be obtained on pure aluminum. </p >
In practice, MAO is most often used for applying thick coatings, because for thin, anodizing is much more cost-effective. Using the MAO method, solid and electrically insulating coatings are obtained, including on parts of complex configuration.
Advantages of micro-arc oxide coatings:
- No special preliminary surface preparation: degreasing, etching, brightening (the electric discharge itself cleans the surface);
- Environmental friendliness, due to the absence of wastewater from preparatory operations;
- The ability to obtain thick (up to 400 microns) coatings, while the electrolyte does not need to be cooled;
- High wear resistance of coatings;
- The porosity of the obtained MAO coatings can be reduced by additional processing to 2-3%.
Disadvantages of micro-arc oxide coatings:
- The use of special current sources that provide high operating voltage and current density;
- Increased power consumption, which, together with point 1, makes it difficult to use this method for processing large aluminum parts;
- Surface roughness increases significantly during coating formation.

Do you want to become our client?
Just leave your request by filling out the form on the right and we will contact you as soon as possible. Thank you!

By submitting an application, you agree to processing of your personal data. Your data is protected.




