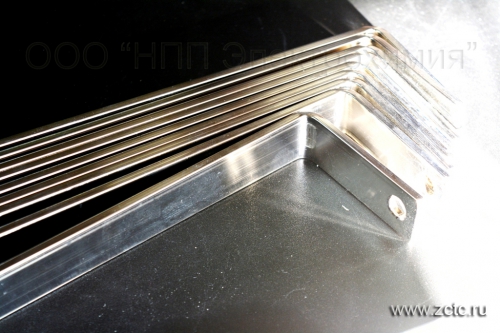
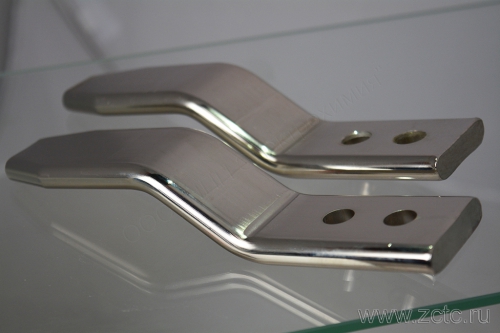
TIN plating of flexible connections and copper busbars up to 1.1 m. BRILLIANT
Description
Copper is an excellent conductor. However, it is easily oxidized in air or in an aggressive environment - a film of oxides, hydroxides and sulfides constantly grows on its surface. This film increases the transition resistance of the current-carrying surface - busbar, contact, terminal. Sometimes the film becomes so dense that the surface can no longer conduct electrical current at a given voltage. Then the tire needs to be stripped, after which the process is repeated.
Tinning a copper busbar with a tin-bismuth alloy allows you to solve this problem. And although the contact resistance of the coating is slightly higher than that of pure copper, it is stable over time. If movable or detachable contacts are switched to a copper bus, then to impart wear resistance, instead of a tin-bismuth coating, you can use tin-nickel alloy . In all cases, the tire acquires a beautiful shiny appearance.
Sometimes aluminum, steel and galvanized contacts are attached to the copper busbar. Their contact with pure copper can lead to the formation of a galvanic couple. In it, copper will be the cathode, and iron, zinc and aluminum will be the anode. The anodes will oxidize, and the contacts themselves will heat up. All this will lead to a voltage drop in them and the risk of fire. The tin coating reduces the potential difference in the galvanic couple and helps protect the contacts.
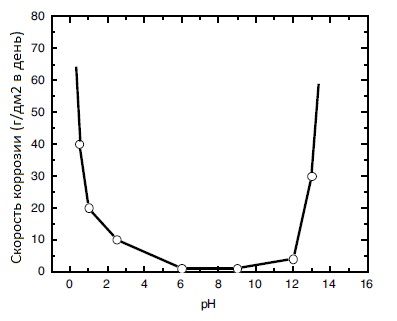
The corrosion rate of pure tin depending on the pH of the environment is shown in the figure:
A separate problem is the tin coating of flexible connections. Flexible connections contain a huge number of cavities in which technological solutions can be retained. For this reason, even after a high-quality coating, corrosion processes may begin immediately or over time. Corrosion destroys the coating, deteriorating the appearance of products and their electrical characteristics. Therefore, the coating of flexible connections differs technologically from the coating of tires and is a much more labor-intensive process. Coating of flexible connections without leakage of solution from cavities has been successfully implemented at our enterprise.
Selective (partial) tinning of individual surfaces of the busbar or flexible connection is possible.
You can order shiny tinning of copper with a tin-bismuth alloy in accordance with GOST 9.305-84 by phone and email specified in the section "CONTACTS".
Characteristics
|
Designation (examples) |
Sn-Bi |
|
Thickness |
3-100 microns (optimal, greater thickness is possible) |
|
Microhardness |
118-198 MPa |
|
Electrical resistivity at 18° C |
11.5⋅10-8 Ohm⋅m |
|
Permissible operating temperature |
200° C |
|
Bismuth content in O-Vi alloy |
0.2-2% |
Advantages of tin plating:
- The tin-bismuth alloy protects the surface of current-carrying busbars from oxidation, thereby stabilizing the transient electrical resistance. Tin is an anode to copper, so it will protect it from corrosion in aggressive environments electrochemically. And given that tin is very prone to passivation, it itself will not actively oxidize. The thinnest oxide film on tin is dense and, once formed, will inhibit the corrosion process of the coating. An increase in corrosion resistance results from the deposition of a shiny tin coating instead of a matte one, which is explained by the lower porosity of shiny coatings.
- It solders perfectly. A shiny coating retains this ability for a longer time than a matte coating, and alloying the coating with bismuth allows it to maintain solderability for longer than 1 year;
- Resistant to sulfur-containing compounds and can be used on parts in contact with all types of plastics and rubbers. Pure copper interacts well with sulfur-containing reagents, forming a poorly conductive layer of copper sulfides.
- The coating is elastic, withstands bending, stretching, flaring, stamping, and press fit. It is well preserved during screwing and seals threaded connections.
- Shiny tin coating is non-porous with a layer thickness greater than 5 microns, unlike matte. The porosity of coatings with a thickness of up to 5 microns can be reduced by reflow.
- Alloying with bismuth helps to avoid destruction of the coating at temperatures below -30°C. The destruction of tin coatings without bismuth occurs due to the transition of compact white tin (β-Sn) to powdery gray tin (α-Sn) (the phenomenon of “tin plague”). This property is very important when delivering equipment to the conditions of the far north.
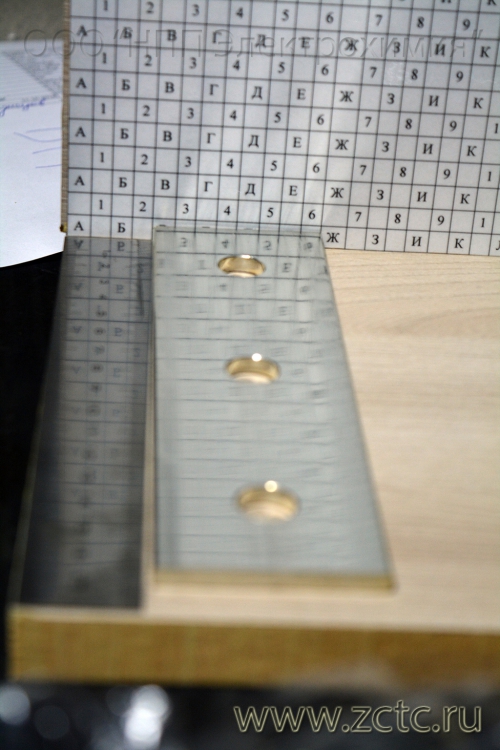
- High uniformity in thickness, even on complex-profile tires, which is confirmed by metallographic studies:
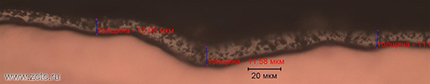
Disadvantages of tin plating:
- Low wear resistance of tin.
- Lower ductility compared to tin-lead.
- Lower antifriction properties compared to tin-lead and lead.
- The coating is unstable in an alkaline environment (although corrosion is extremely slow at room temperatures).

Read also articles
Galvanizing mechanism
Description of the galvanic galvanizing process. Electrolytes.
Hydrogenation and removal of hydrogenation of steel after galvanizing
The problem of hydrogenation during galvanizing. How to dehydrate?

Do you want to become our client?
Just leave your request by filling out the form on the right and we will contact you as soon as possible. Thank you!

By submitting an application, you agree to processing of your personal data. Your data is protected.