
 Fazlutdinov K.K.
Fazlutdinov K.K.
 01.06.2016 (updated 22.06.2021)
01.06.2016 (updated 22.06.2021)
 43519 views
43519 views
Mechanism and technology of galvanic galvanizing. Structure and properties of galvanic coatings.
Content:
1. What is zinc and what are its corrosion characteristics?
2. Electrolytes for galvanizing.
1. What is zinc and what are its corrosion characteristics?
- Zinc is a light gray metal with a bluish tinge.
- The melting point is 419.5°; C, and the density is 7.133 g/cm3. In a cold state, zinc is brittle, and at a temperature of 100-150 ° C it is very ductile, bends well and easily rolls into sheets and foils up to hundredths of a millimeter thick. At >250° With it, it becomes brittle again and easily turns into powder.
- Metal can be soldered using active fluxes, for example, ZnCl2.
- It has an average hardness, which largely depends on the method of its production and purity. The hardness of zinc coatings ranges from 0.4 to 2.0 GPa.
- The pH of the medium has a great influence on the corrosion rate of zinc. In the pH range of 7-12, zinc practically does not dissolve. The corrosion rate increases when deviating from the specified values.


Zinc plating is the most common way to coat steel and cast iron to protect it from atmospheric corrosion. Approximately 40% of world zinc production is spent for these purposes.
Zinc coating — is the application of a thin layer of zinc to a product to give it the required characteristics (corrosion protection, color, gloss, wear resistance, etc.).</ p>
Zinc coating is widely used due to anodic nature of protection. The potential of zinc is - 0.763 V, which is more negative than the potential of ferrous metals: steel, iron, cast iron, so the coating protects them from corrosion by electrochemical means. The protective properties of coatings are preserved even with a small layer thickness, as well as in the presence of pores and exposed areas. Numerous examples of the protective effect of the coating on bare sections of steel are known, for example, the cut edges of galvanized iron, the cross section of the wire, the uncoated thread of the nut if it is screwed onto a galvanized screw, etc.
|
Designation |
Ts.hr - rainbow (yellow) C.hr.btsv - colorless (white) Ts.hr.khaki - khaki (olive) Ts.hr.h - black C.phos - with phosphating Zink coating - English. designation |
Thickness |
6-50µm (greater thickness possible) |
Microhardness |
490-1180 MPa |
Electrical resistivity at 18oC |
5.75×10-8 ohm⋅m |
Permissible operating temperature |
300°C |
The anodic nature of steel coating protection in some cases can be replaced by a cathodic one, and then corrosion occurs very intensively. A similar effect is observed under the influence of hot water at temperatures above 70 ° C (boiler plants, autoclaves). In dry air at room temperature, zinc almost does not oxidize. Starting from a temperature of 225°C, the rate of oxidation in air increases rapidly.
In humid air and sea water, especially in the presence of CO2 and SO2, zinc degrades rapidly even at room temperature. temperature, being covered with a surface film of basic hydrocarbonates. As corrosion products accumulate on the surface and partially fill the pores with them, the corrosion rate of zinc decreases, and the film serves as additional protection. In hot water, pitting can begin, with white cup-like deposits forming around gas bubbles.
The rate of zinc corrosion is especially significant in the atmosphere of industrial cities and in the tropics.
When heated strongly in air, especially in the presence of CO2, zinc burns to form zinc oxide. Zinc readily dissolves in solutions of strong acids to form the corresponding salts and hydrogen.
Reacting with dilute acids HCl and H2SO4 releases hydrogen:
Zn + 2H+ → Zn2+ + H2
and with HNO3 - nitrogen oxides.
Strong alkali solutions oxidize zinc to form water-soluble zincates. Chemically pure zinc, in contrast to that contaminated with impurities of other metals, dissolves slowly in acids and alkalis. This is due to the fact that hydrogen, which should be released during this reaction, has a high overvoltage.
Zinc has low chemical resistance to volatile products released during aging of organic materials such as synthetic resins, drying oils, chlorinated hydrocarbons. Zinc coatings are easily destroyed if they are in contact or in a closed volume with freshly painted or oiled parts.
Thus, the protective effect of the coating is determined primarily by its thickness, which depends on the operating conditions of the products. Next, we will talk about galvanizing.
|
Characterization of operating conditions |
Thickness, microns |
Covering designation by GOST 9.306-85 |
Operation in heated and ventilated rooms with an air temperature of 25±10°C and a humidity of 65±15% |
6-9 |
D.6 |
Operation under a canopy and in unheated rooms; lack of influence of atmospheric precipitation; the atmosphere is polluted with small amounts of industrial gases; air temperature from -60 to +60°С, relative humidity 95±3% |
15-18 |
C.15.chr |
Outdoor use; exposure to precipitation, fog; the atmosphere is polluted with industrial gases, dust; ambient temperature from -60 to +80°C, relative humidity 95±3% |
24-30 |
C.24.chr |
Operation in special conditions |
36-42 |
C.36.xp |
Note: xp - chromate coating treatment |
||
Protection properties can be greatly increased in a variety of ways, the most common being:
- formation of chromate films on the surface of zinc by chemical treatment of galvanized parts in solutions containing chromic acid or its salts; this operation is called passivation or chromating;
- formation of phosphate films on the coating as a result of processing parts in solutions containing salts of phosphoric acid;
- applying additional paintwork, with better results if the paintwork operation is preceded by phosphating.
2. Coating electrolytes.
Quality coatings is largely determined by the nature of the electrolyte used.

Electrolytes for zinc plating can be divided into two main groups:
- Simple acidic acids (sulfate, chloride, hydroboron fluoride), in which zinc is in the form of hydrated ions;
- Complex complexes, in which zinc is present in the form of complex ions, negatively or positively charged. Of the complex electrolytes, cyanide, zincate, ammonia, pyrophosphate and others are known.
The quality of the deposits on the cathode and the rate of the deposition process depend on the nature and composition of the electrolytes. Since the quality of deposits and the rate of the process are largely determined by the nature and degree of change in cathode potentials, for a comparative assessment of zinc plating electrolytes (as well as other types of metal coatings), it is best to proceed from the relative position of the polarization curves. The higher the cathodic polarization, the more fine-grained and uniform in thickness deposits on the cathode.
A comparison of the polarization curves shows (Figure 1) that the lowest polarization is characteristic of the galvanizing process in sulfate electrolyte, the highest - in cyanide and close to it zincate.
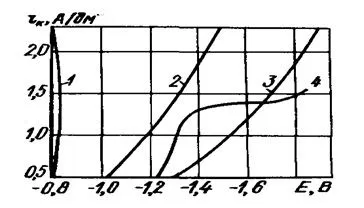
Figure 1 — Graph of the cathodic polarization of zinc electrolytes: 1 - sulfate; 2 - ammonium; 3 - cyanide; 4 - zincate.
In the first case, an increase in current density is almost not accompanied by a change in the current output of the metal, in contrast to alkaline solutions, especially cyanide solutions, where the current output decreases with increasing current density. Therefore, acidic electrolytes are suitable for galvanizing parts of a simple configuration, tape, wire. They allow the use of higher current densities than cyanide ones and, therefore, are distinguished by a higher rate of coating growth. The deposition of zinc from complex electrolytes proceeds with a high scattering power, so these electrolytes provide not only fine-grained, but also uniform coatings on parts, both simple and complex.
The overvoltage of hydrogen on zinc reaches a significant value: at a cathode current density of 1 A/dm2 it is 0.75 V, and at 3 A/dm 2 — approaches 1 V. In this regard, the cathodic current efficiency of zinc in simple electrolytes reaches 96-98%; therefore, the predominant discharge of zinc ions occurs at the cathode.
When coating in complex electrolytes, zinc and hydrogen are released together. The rate of hydrogen evolution increases as the current density increases, since this increases the potential for zinc evolution. The release of hydrogen leads to a significant hydrogenation of products, which worsens their mechanical properties - plasticity decreases and the tendency of steel to brittle fracture increases. Therefore, in electrolytes with low current efficiency, it is not allowed to apply zinc to parts made with a tensile strength of 1400 MPa or more.


2.1 Simple acidic electrolyte.
These electrolytes are the most widely used in the industry. Using them allows you to precipitate zinc at a high rate. Acidic electrolytes are stable in operation, highly productive, and relatively cheap.
Suitable-looking precipitation can be obtained from simple acidic electrolytes containing only a zinc salt and a small amount of sulfuric acid. However, in practice, to improve the quality of the coating, surfactants are usually added to the salt solution, as well as alkali metal salts and substances imparting buffer properties to the electrolyte.
Basic cathode reaction:
Zn2+ + 2e = Zn
Zinc concentration is chosen depending on the required process speed. The higher the concentration in the solution, the higher the allowable current density, but the less uniform the thickness of the zinc deposits. Solutions with zinc salt concentration from 20-30 to 700-800 g/l can be used for galvanizing parts. Highly concentrated electrolytes are used in continuous galvanizing units for strip, wire and pipes.


Electrolytes with pH = 4-5 are practically used, since with a high acidity of the solution, the current output at the cathode is greatly reduced due to hydrogen evolution, and the current output at the anode increases over chemical dissolution of zinc. Neutral zinc solutions are also not suitable for galvanizing, since as a result of hydrogen evolution and alkalization of the medium at the cathode, hydroxides are formed, polluting the deposit and deteriorating the quality of the coating.
To maintain a pH of about 4.5, buffer additives are introduced into the electrolyte - acetic, more often boric acid (20-30 g / l). Instead of acetic acid, it is advisable to introduce sodium acetate, which, after the addition of sulfuric acid, gives an equivalent amount of weakly dissociated acetic acid. An electrolyte containing about 30 g/l of aluminum sulfate or potassium alum has good buffering properties. In the presence of aluminum salts at pH = 4.5, the cathodic polarization increases (Figure 2) and the precipitates are light, semi-shiny, fine-grained.
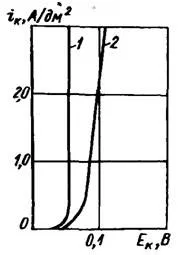
Figure 2 — Effect of aluminum sulfate on polarizability: 1 — no additives; 2 — in the presence of Al2(SO4)3.
The buffering properties of aluminum sulfate are based on the fact that at pH=4-4.5 it undergoes hydrolysis to form H2SO4< /sub>
Al2(SO4)3 + 6H2 O ↔ 2Al(OH)3 + 3H2SO4
Sometimes, salts of other metals that are not released at the cathode are added to the sulfate electrolyte, for example, sulfates or sodium and ammonium chlorides (up to 2 g-eq / l or more ), mainly to increase the electrical conductivity of solutions. When sulfates are added, cathodic polarization increases, which helps to improve the distribution of the metal over the cathode surface.
In cases where increased requirements are imposed on the appearance, corrosion resistance, macro- and microdistribution of a zinc coating, multicomponent organic brightening agents containing additives to increase scattering, covering and leveling power, deposition rate of the coating and its gloss. There are brighteners that make it possible to obtain shiny coatings that are quite uniform in thickness and level the surface microprofile at high current densities (up to 10 A/dm 2) on parts of medium configuration from acidic electrolytes. . Dextrin, glucose, gelatin, wood glue, phenols, glycerin and others are widely used as additives to acidic electrolytes.
Which impurities are harmful in an acidic electrolyte?
Harmful impurities in acidic electrolytes are salts of metals more electropositive than zinc, for example, copper salts (0.01 g / l), arsenic (0.001-0.005 g / l), antimony (0.001-0.01 g / l), lead, all salts of nitric acid and some organic substances (turpentine, acetone, glue), etc. In the presence of small amounts (fractions of a gram per liter) of electropositive metals in acidic zinc spongy deposits form on the cathode due to the release of these metals at the limiting diffusion current.
- Lead, present in zinc sulfate electrolyte, in the absence of chlorides and dextrin, does not affect the quality of zinc deposits due to the low solubility of lead sulfate, which in a neutral aqueous medium is approximately 0.01 g / l (counting for metal).< /li>
- In a sulfate electrolyte containing dextrin additives, and in electrolytes containing chlorine ion, the zinc deposit on the cathode darkens already at a lead concentration of about 0.05 g / l, and at a concentration of 0.3 g / l and higher on the surface cathode, a spongy black precipitate is formed.
- Tin at concentrations up to 0.3 g/l does not affect the appearance of the coating. With an increase in the content of tin to 1 g/l at a current density of about 100 A/m2, the cathode deposits become dark and loose, which is explained by the reduction of tin ions at the limiting diffusion current. Iron has a great influence on the quality of zinc deposits in electrolytes with organic additives.
- To remove impurities of electropositive metals, the pre-acidified electrolyte is processed with direct current at a low current density.
- The iron salts are removed as hydroxide Fe(OH)3 after neutralizing the solution with sodium bicarbonate and adding hydrogen peroxide or alkali metal persulfate while heating to 70-100°C. After settling the Fe(OH)3 precipitate, the solution is decanted or filtered.
- In the presence of nitrates, spongy precipitates are formed on the cathode, including zinc hydroxide, the formation of which is explained by the reduction of NO3- to ammonia and hydroxylamine and, as a result, alkalization of the cathode layer. The sponge is eliminated only with a strong acidification of the electrolyte, which, at low current densities, causes a significant decrease in the current efficiency.
To remove harmful organic impurities, depending on the nature of these impurities, the electrolyte is worked through with direct current with lead anodes (in the absence of a chloride ion in the solution) at ia = 500-1000 A/m2, treatment with manganese peroxide, activated carbon, etc.
The temperature of acidic electrolytes is usually kept within 18-25°C. During electrolysis with high current densities (>5*102 A/m2) in electrolytes that do not contain organic additives (for example, when galvanizing wire, strip, sheets), the temperature is raised to 50 °C.
Current densities at the cathode in unmixed electrolytes are no higher than 200-300 A/m2. When mixing the electrolyte with compressed air, the permissible upper current density limit can be significantly increased depending on the composition and temperature of the electrolyte, the type of products being coated (parts, wire, tape, sheets).
Permissible current densities are significantly increased (up to 200-500 A/m2) and the decorative appearance of zinc deposits is improved during electrolysis using ultrasound. Cathodic current outputs range from 95-100% depending on pH, t and ik.
Anodes for zinc plating in acid electrolytes are usually made from pure electrolytic zinc (99.8-99.9% Zn), which can contain no more than 0.03% lead, 0.02% cadmium, 0.002% copper, 0.04% iron and 0.001% tin.
In all acidic electrolytes, anodes dissolve with high current efficiency, which at pH-1-2 is more than 100% due to corrosion.
Anodes should be sheathed with filter cloth or chlorine to avoid contamination of the electrolyte by anode sludge. It is recommended to use zinc containing 0.05-0.2% magnesium and 0.25-1% calcium. Anodes made of such zinc form sludge to a lesser extent and dissolve with a low current efficiency, due to which the electrolyte is more stable.
Recently, cast anodes of various configurations have become widespread: in the form of balls, cylinders, etc., which are loaded into titanium mesh baskets. The use of anodes of this shape makes it possible to use the metal more fully and reduce its consumption compared to lamellar anodes.
Approximate compositions and modes of operation of acidic electrolytes are given in Table 2.
|
Electrolyte components |
The composition of electrolytes |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
ZnSO4·7H2O |
215 |
200-250 |
140 |
70-100 |
─ |
450-700 |
─ |
ZnCl2 |
─ |
─ |
─ |
─ |
─ |
─ |
200-250 |
Zn(BF4)2 |
─ |
─ |
─ |
─ |
250-300 |
─ |
─ |
Al2(SO4)3·18H2O |
30 |
25-30 |
─ |
─ |
─ |
25-30 |
─ |
Na2SO4·10H2O |
50-100 |
─ |
70 |
─ |
─ |
─ |
─ |
NH4Cl |
─ |
─ |
─ |
200-250 |
─ |
─ |
250-300 |
NH4BF4 |
─ |
─ |
─ |
─ |
25-30 |
─ |
─ |
CH3COONH4 |
─ |
─ |
─ |
30-40 |
─ |
─ |
─ |
NaCl |
─ |
─ |
25 |
─ |
─ |
─ |
─ |
H3BO3 |
─ |
25-30 |
─ |
─ |
10-15 |
─ |
─ |
Dextrin |
10 |
8-10 |
─ |
─ |
─ |
─ |
─ |
Dispersant NF |
─ |
─ |
─ |
50-100ml/l |
─ |
─ |
─ |
DCU |
─ |
0,5-1 |
2 |
─ |
─ |
─ |
─ |
U-2 |
─ |
1-1,5 |
─ |
─ |
─ |
─ |
─ |
OP-10 |
─ |
─ |
─ |
─ |
8-10 |
─ |
─ |
Thiocarbamide |
─ |
─ |
─ |
─ |
2-3 |
─ |
─ |
pH |
3,8-4,5 |
4-4,2 |
2 |
3,5-5 |
3-4,5 |
3,5-4,5 |
3,8-5 |
t, oC |
18-25 |
15-30 |
20 |
18-25 |
20 |
40-50 |
18-65 |
ik, A/dm2·10-2 |
0,5-2 |
1-3 |
2 |
1-3 |
до 4-5 |
10-300 |
10-100 |
||||||
At cathodic current density over 200 A/m2 all electrolytes should be agitated with compressed air free of dust and oil and filtered continuously or intermittently . Electrolyte 2 is recommended for obtaining shiny zinc coatings on products of a simple configuration with an anode and cathode surface ratio Sa : SK = 2 : 1.
Electrolyte 3 produces shiny precipitates at an ACC concentration of at least 4 g/l. Electrolyte 6 is recommended to be used with intensive mixing with compressed air and continuous flow (with filtering) for galvanizing sheets, moving (continuously) wire and tape.
The smaller the diameter of the wire and the width of the tape, the greater the allowable current density. Electrolyte 7 is used for the same purposes as electrolyte 6, but at lower allowable current densities.
Thus, despite all the positive aspects of acid electrolytes, namely, stability in operation, high performance, relative cheapness, low hydrogenation of steel parts, they also have a number of disadvantages, the main of which is low scattering power. However, in recent years, due to the increased requirements for environmental safety of technological processes in general, and galvanic processes in particular, electrolytes based on zinc chloride with brightening additives have been proposed as more promising for obtaining shiny zinc coatings.
2.2 Weakly acid electrolytes based on zinc chloride.
Weakly acid galvanizing electrolytes based on zinc chloride are a special group. These electrolytes are characterized by high electrical conductivity (twice higher than sulfate electrolytes) and the intensity of the process. Hydrogen is released in small amounts, i.e. low hydrogenation of parts, which means that hydrogen embrittlement does not occur. However, the presence of chlorides in the electrolyte causes intense corrosion of the equipment, so bathtubs, side suctions, filters, pumps, valves and other auxiliary equipment in contact with the electrolyte are made of corrosion-resistant materials. For these purposes, polypropylene, polyvinyl chloride, fluoroplast and a number of other engineering plastics are widely used. In addition, chloride residues in the pores of the coating can contribute to increased corrosion of the coating, so the washing of parts must be organized very well.
Coatings made from weakly acidic electrolytes have low internal stresses, which reduces the tendency of the coating to break.
In connection with the indicated features, weakly acidic electrolytes are recommended for galvanizing small parts in drums and in conveyor installations. Some compositions of weakly acidic electrolytes are shown in Table 3.
|
Components
|
Electrolyte 1 |
Electrolyte 2 |
Electrolyte 3 |
|
ZnCl2 |
60-120 |
20-70 |
100-120 |
|
КСl(NaCl) |
180-230 |
200-250 |
200-230 |
|
H3BO3 |
15-30 |
15-30 |
- |
|
NH4Cl |
- |
- |
20-25 |
|
Limeda NC-10 |
30-70 |
20-70 |
- |
|
Limeda NC-20 |
2,5-5,0 |
2,5-10 |
- |
|
ik, A/dm2 |
0,5-5,0 |
0,5-1,5 |
50 |
|
t, °С |
15-30 |
15-30 |
40-60 |
|
pH |
4,5-6 |
4,5-5,8 |
3-4 |
In the practice of galvanizing sheet steel and wire, electrolyte 3 is used. However, the big disadvantage of this electrolyte is its high the concentration of ammonium ions, which greatly complicates the treatment of wastewater from electroplating industries.
Electrolyte 2 is designed for deposition of zinc in rotary machines. It is recommended to filter it periodically (at least once a day).
Zinc plating of parts using electrolyte 1 requires agitation with compressed air and constant filtration to remove mechanical impurities. The latter is due to the fact that at the sites of deposition of contaminants on the surface, an increase in the local current density occurs, accompanied by an intensive growth of dendrites at this site. Therefore, the higher the current density, the more thorough the cleaning of the electrolyte should be.
At present, a new electrolyte of weakly acidic zinc plating has been developed, in which sodium acetate is introduced as a buffer additive instead of the toxic substance boric acid. This electrolyte has all the advantages of the electrolytes indicated in Table 3, but is more environmentally friendly.

2.3 Complex cyanide electrolytes.
In cyanide electrolytes, zinc occurs as complex anions Zn(CN)42- and Zn(OH)42-. They are formed by reactions:
Zn(OH)2 + 4NaCN = Na2[Zn(CN)4] + 2NaOH
ZnO + 2NaOH + H2O = Na2[Zn(OH)4]
Осаждение же цинка происходит по схеме:
Na2[Zn(CN)4] ↔ 2Na+ + Zn(CN)42-
Zn(CN)42- + 2е → Zn + 4OH-
а при избытке щелочи:
Na2[Zn(OH)4] ↔ 2Na+ + [Zn(OH)4]2-
[Zn(OH)4]2- + 2е → Zn + 4OH-
Due to the high dissipation power and stability in operation, the cathode current efficiency in cyanide electrolytes reaches 50-80%. Cyanide electrolytes are widely used in industry for galvanizing products of complex shape.
The amount of zinc cyanide and sodium zincate depends on the amount of sodium cyanide and sodium hydroxide in the electrolyte. Precipitation occurs from two compounds at the same time.
Excessive cyanide is needed to increase the dissipation power, while increasing cyanide content reduces cathode current efficiency.
Caustic soda is introduced into the electrolyte to expand the range of operating current densities, increase electrical conductivity, and also to prevent the formation of hydrocyanic acid.
Glycerine is added to improve the texture of the coating and produce semi-shiny deposits. The purpose of sodium sulfide is to precipitate in the form of insoluble sulfides of heavy metal cations that accidentally enter & nbsp; into the electrolyte.
The composition of cyanide baths is shown in Table 4.
|
Components and mode of operation |
Electrolyte number |
1 |
2 |
3 |
Components g/l |
|
zinc oxide |
20-45 |
8-10 |
20-30 |
Potassium cyanide |
- |
- |
60-80 |
sodium cyanide |
50-120 |
18-20 |
- |
Sodium hydroxide |
50-100 |
60-80 |
- |
caustic potash |
- |
- |
75-110 |
Potassium sulfide |
0,5-5,0 |
- |
3-7 |
Glycerol |
3-5 |
- |
3-5 |
Potassium metatitanate |
- |
- |
0,7-1,0 |
Working mode |
|
Electrolyte temperature, ºС |
15-20 |
15-25 |
15-25 |
Current density, A/dm2: |
|
Without mixing |
1,0-3,0 |
0,5-2,5 |
1,0-3,0 |
With stirring |
До 8,0 |
- |
До 4 |
current output, % |
60-80 |
70-85 |
70-90 |
||||||||
Electrolyte 1 is designed to cover parts on hangers. Electrolyte 2 has a number of advantages. It has good hiding power, is less sensitive to heavy metal impurities, and requires less wastewater treatment costs.
2.4 Ammonia (chlorammonium) electrolytes.
In order to replace toxic cyanide electrolytes and reduce the cost of treating cyanide-containing wastewater, ammonia electrolytes, in which zinc is in the form of a complex cation such as Zn(NH 3)n(H2O)m2+, where
n=2 in acidic environment, n=4 in alkaline environment.
Ammine zinc compounds are obtained by reacting zinc oxide with ammonium salts according to the reaction:
ZnO + nNH4Cl = Zn(NH3)nCl 2 + H2O
Precipitation is by reaction:
[Zn(NH3)n]2+ + 2e + nH2 O = Zn + nNH4+ + nOH-
The good scattering power of the electrolyte, close to that of cyanide electrolytes, is also due to their high electrical conductivity. The specific electrical conductivity of ammonia electrolytes is 30-40% higher than that of cyanide electrolytes. The increased electrical conductivity of the electrolyte plays an important role when coating parts in bells or drums, as it is possible to conduct the process at a voltage of 5 V. Ammine electrolytes are practically harmless to workers servicing the baths, stable in operation and easily corrected.
Ammine electrolytes have a number of technical advantages:
• With their help, cast irons are coated more easily;
• Thin-walled and heat-treated parts are not subject to hydrogenation due to high current efficiency.
Buffer compounds are introduced into electrolytes to stabilize the pH value in the cathode zone. Boric acid or acetic acid salts are used as buffer compounds. The pH value of electrolytes has a great influence on the scattering power and structure of coatings. As the pH increases, the scattering power improves. Unlike cyanide, ammonia electrolytes are less sensitive to the ingress of organic impurities into them. Since they are slightly alkaline or almost neutral, they do not destroy the insulating materials applied to the hangers or to the surface of the parts to be coated.
|
Components and mode of operation |
Electrolyte number |
|||
|
1 |
2 |
3 |
4 |
|
|
Components, g/l |
|
|||
|
Zinc oxide |
10-20 |
40-60 |
35-40 |
- |
|
Zinc sulfate |
- |
- |
- |
80-100 |
|
Ammonium chloride |
200-300 |
240-250 |
200-220 |
200-250 |
|
Boric acid |
25-30 |
- |
- |
- |
|
Urotropin |
- |
40-60 |
20-25 |
- |
|
Ammonia water 25% |
- |
100-120 |
- |
- |
|
Skin glue |
1-2 |
2-4 |
- |
- |
|
Preparation OS-20 |
- |
- |
4-5 |
- |
|
Dispersant NF, ml/l |
- |
- |
6-8 |
17-35 |
|
Working mode |
|
|||
|
рН |
5,9-6,5 |
8,0-8,4 |
7,8-8,2 |
3,5-4,5 |
|
Temperature, ºС |
15-25 |
15-25 |
15-25 |
18-25 |
|
Current density, A/dm2 |
0,5-1,0 |
1,0-2,0 |
2,0-3,0 |
3,0-5,0 |
|
Current output, % |
95-98 |
96-98 |
94-98 |
- |
Electrolyte 1 is cheaper and simpler in composition, recommended for coating parts with a simple profile and parts in bell and drum baths.
Electrolyte 2 has the highest scattering power and is recommended for covering complex profiles.
Electrolyte 4 is used only for coating small parts in drums and bells, as intensive dissolution of anodes and abundant sludge formation occur in stationary baths.
The main disadvantage of ammonia electrolytes is the inevitable presence of ammonium salts in wastewater, which is unacceptable according to modern sanitation requirements.
2.5 Alkaline Zinc Electrolytes.
Zincate electrolytes, like ammonia electrolytes, are used to replace cyanide electrolytes. The high scattering power of zincate electrolytes is due to the good electrical conductivity of the electrolyte. A significant effect on the increase in dissipation has a decrease in current efficiency with an increase in current density.
The kinetics of the cathode process in galvanizing with zincate alkaline electrolyte is much more complex than is usually presented in educational materials and technical literature. Usually we are talking about reactions:
ZnO + 2NaOH + H2O = Na2[Zn(OH)4< /sub>] (at the time of electrolyte preparation)
Na2[Zn(OH)4] ↔ 2Na+ + [Zn(OH)4]2-
[Zn(OH)< sub>4]2- + 2e → Zn + 4OH-
However, other zinc complexes can also be present in the bulk of the solution, and the cathode discharge can even come from electrically neutral particles.
It is important to note that the composition and structure of Zn(II) complexes in the bulk of the electrolyte differs from those on the cathode surface during the reaction. Thus, the [Zn(OH)4]2- complex (sodium tetrahydroxozincate) dominates in the volume at a high alkali concentration. In a low alkaline medium, in addition to this complex, particles [Zn(OH)]+, [Zn(OH)2] and [Zn(OH)3 >]-. They, respectively, contain less than 4x OH- groups. Such data were obtained by the methods of measuring the equilibrium potential, vibrational spectroscopy, NMR spectroscopy, and the solubility method. The results are beyond doubt.
Initially, it was believed that on an amalgamated zinc electrode in a zincate solution, zinc is reduced by the reaction:
Zn(ОН)2 + 2е → Zn + 2ОН-
This has been pointed out by many researchers. In such a mechanism, two electrons are transferred to an electrically neutral particle. However, from the modern standpoint of the theory of polar solvents, this is unlikely; charge transfer along one electron is much more likely. In this section, there are several multi-stage theories based on the results of chronopotentiometry, taking potentiodynamic curves and the temperature-kinetic method.
The first mechanism for the discharge of zinc from sodium tetrahydroxozincate is the sequential flow of chemical, electrochemical, chemical and again electrochemical stages:
[Zn(ОН)4]2-∙4Н2О ↔ [Zn(ОН)2∙2Н2О] + 2OH- + 2Н2О
[Zn(ОН)2∙2Н2О] + е ↔ [Zn(ОН)2]-∙2Н2О
[Zn(ОН)2]- ∙2Н2О ↔ [ZnОН] + ОН- + 2Н2O
[ZnОН] + e → Zn + ОН-
This mechanism is otherwise called CECE.
When a complex with three OH- groups is discharged, the sequence is modified:
[Zn(OH)3]- ↔ [Zn(OH)2] + OH-
[Zn(OH)2] + e ↔ [ZnOH] + OH-
[ZnOH] + e ↔ Zn + OH-
In both cases, the discharge comes from an electrically neutral zinc hydroxide particle [Zn(OH)2].
Bockris refined the sodium tetrahydroxozincate discharge mechanism by assuming that the first electron would go to [Zn(OH)3]-, and not on [Zn(OH)2∙2H2O]. In this case, there is no stage of discharge of neutral [Zn(OH)2]:
[Zn(OH)4]2- ↔ [Zn(OH)3]- + OH-
[Zn(OH)3]- + e → [Zn(OH)2]- + OH-
[Zn(OH)2]- ↔ [ZnOH] + OH-
[ZnOH] + e ↔ Zn + OH-
Electron addition step to [Zn(OH)3]- here limiting.
Another mechanism for discharging sodium tetrahydroxozincate is in three stages:
[Zn(OH)4]2- ↔ [Zn(OH)2] + 2OH-
[Zn(OH)2] + e → [Zn(OH)] + OH-
[ZnOH] + e ?Zn + OH-< br />
Ultimately, in all experimental models, the coordination number of the central zinc atom decreases by OH groups, and the final discharge of zinc comes from the neutral particle [ZnOH], where zinc has a valency (I).
Compositions of zincate electrolytes are shown in Table 6.
|
Components and mode of operation |
Electrolyte number |
||
|
1 |
2 |
3 |
|
|
Components, g/l (ml/l) |
|
||
|
Zinc oxide, g/l |
10-17 |
12-15 |
12-15 |
|
Caustic soda, g/l |
90-120 |
100-120 |
80-100 |
|
Glitter 1, ml/l |
9-11 |
- |
- |
|
Glitter 2, ml/l |
9-11 |
- |
- |
|
PEPA, g/l |
- |
2-4 |
- |
|
Triethanolamine, ml/l |
- |
- |
20-30 |
|
Thiourea, g/l |
- |
0,5 |
- |
|
Polyepoxyamine, ml/l |
- |
- |
1,5-2,5 |
|
p-Dimethylaminobenzaldehyde, g/l |
- |
- |
0,2-0,3 |
|
Working mode |
|
||
|
Temperature, ºС |
20-30 |
18-25 |
18-25 |
|
Current density, A/dm2 |
|
||
|
Without mixing |
1-4 |
1-2 |
1-4 |
|
With stirring |
- |
- |
5-6 |
Surfactants must be introduced into electrolytes, without which it is impossible to obtain a high-quality compact zinc precipitate. The same surfactants, being sorbed on the cathode surface, reduce the overvoltage of hydrogen and contribute to an increase in its amount released at increased current densities.
Electrolyte 1 and 3 provide brilliant zinc coatings. Electrolyte 2 makes it possible to obtain light semi-brilliant zinc deposits.
2.6 Pyrophosphate electrolytes.
To replace toxic cyanide electrolytes, pyrophosphate electrolytes are also recommended, in which zinc is in the form of a pyrophosphate complex of the anion [Zn(P2O7< /sub>)2]6-.
Pyrophosphate electrolytes are relatively harmless, stable in operation and characterized by high scattering power. However, due to the poor solubility of zinc pyrophosphates, especially sodium salts, the process is carried out with the obligatory heating of the electrolyte at relatively low current densities. Zinc anodes are also poorly soluble in pyrophosphate electrolyte due to the formation of hardly soluble films on the surface. In addition to pyrophosphates, phosphates are introduced into the composition of electrolytes in order to impart buffer properties to the electrolyte. In order to make the coating shine, organic additives are introduced into the electrolyte: saccharin, vanillin, furfural. The cathode current efficiency is 65-90%, and a sharp decrease occurs with an increase in current density up to 2.5 A/dm2.
Compositions of pyrophosphate zinc baths are shown in Table 7.
|
Components and mode of operation |
Electrolyte number |
|
|
1 |
2 |
|
|
Components, g/l |
|
|
|
zinc sulfate |
50-90 |
60-70 |
|
Potassium pyrophosphate |
250-350 |
300-330 |
|
Ammonium sulfate |
15-20 |
- |
|
Disubstituted ammonium phosphate |
- |
45-55 |
|
Ammonium chloride |
20-50 |
- |
|
Dextrin |
1-3 |
- |
|
Skin glue |
3-5 |
- |
|
Sulfanilic acid |
- |
0,1-0,5 |
|
Working mode |
|
|
|
рН |
7,5-8,5 |
8,5-9,0 |
|
current density (A/dm2) при: |
|
|
|
t =18-20ºC |
2,0-3,5 |
1-2 |
|
t = 45-50ºC |
5,0-5,5 |
2-5 |
|
Anode current density, A/dm2 |
0,7-1,2 |
0,5-1,0 |
|
current output, % |
85-95 |
83-92 |
Electrolyte 2 — electrolyte of bright zinc plating, characterized by the greatest ability and recommended for coating complex relief parts.
Anode processes during galvanizing are discussed in detail here.

Do you want to become our client?
Just leave your request by filling out the form on the right and we will contact you as soon as possible. Thank you!

By submitting an application, you agree to processing of your personal data. Your data is protected.




