TIN PLATING OF COPPER PARTS AND CONTACTS. BRILLIANT
Description
Tin and bismuth are soft metals with a silvery-white color under normal conditions. In the alloy, bismuth is capable of forming intermetallic compounds - bismuthides. Tin-bismuth coating has high chemical resistance. Under atmospheric conditions, even in the presence of moisture, it oxidizes slowly. Dilute solutions of mineral acids (sulfuric, hydrochloric, nitric), concentrated alkalis at room temperature react with tin slowly, sulfur compounds have almost no effect. Tin is very stable in organic acids. At the same time, tin dissolves in concentrated sulfuric and hydrochloric acids and concentrated alkalis when heated. Tin is a polymorphic metal. Under normal conditions, it exists in the form of a β-modification (white tin), stable above 13.2 °C. At low temperatures (from -30 to -50°C), white tin transforms into another allotropic modification (gray tin). The transition is accompanied by an increase in specific volume, which leads to the destruction of the metal. This phenomenon was called the “tin plague”. In a tin-copper pair (or its alloys), tin is the anode and protects the copper from corrosion protectively, even if the coating is damaged.
It should be taken into account that diffusion of zinc from brass into a tin coating is possible at room temperature (about a month when stored in a dry heated room) - the coating becomes gray and loses its physical properties -mechanical and electrical properties. Diffusion of copper is also possible, however, experimentally it turns out that it occurs only at high temperatures (above 200°C) with the formation of white bronze. Therefore, when coating brass parts with tin, an underlayer of copper or nickel with a thickness of at least 2 microns is required.
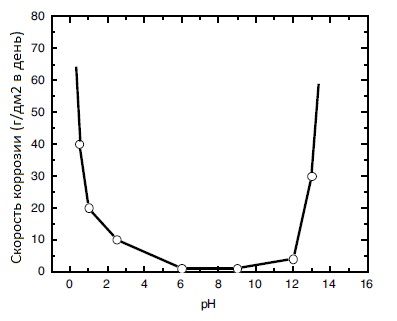
Tinning with a tin-bismuth alloy on the surface of copper and its alloys solves the following main problems: protection against corrosion of current-carrying parts of parts, stabilization of transient electrical resistance, protection from direct contact with aluminum (galvanic couple, which is corrosive to aluminum), improved solderability, facilitating make-up while simultaneously sealing threaded connections. The corrosion rate of pure tin depending on the pH of the medium is shown in the figure:
Selective (partial) tinning of individual surfaces of parts or contacts is possible.
You can order shiny tinning of steel with tin-bismuth alloy in accordance with GOST 9.305-84 by phone and email indicated in the section "CONTACTS".
A microphotograph of a cross-section of the coating is shown below:
Characteristics
|
Designation (example) |
Sn-Bi |
|
Thickness |
3-100 microns (optimal, greater thickness is possible) |
|
Microhardness |
118-198 MPa (12-20 kgf/mm2) |
|
Electrical resistivity at 18° C |
11.5⋅10-8 Ohm⋅m |
|
Permissible operating temperature |
200° C |
|
Bismuth content in O-Vi alloy |
0.2-2% |
Advantages of tin plating:
- Perfectly protects copper from corrosion anodicly (just as zinc protects steel);
- Excellently soldered and allows you to maintain solderability for up to a year;
- Resistant to the effects of sulfur-containing compounds, does not form sulfides in industrial atmospheres, does not change the contact resistance;
- Completely stable in organic and dilute mineral acids (sulfuric, hydrochloric, nitric);
- Elastic. Withstands plastic deformation well;
- Alloying with bismuth helps prevent “needling” and also avoids destruction of the coating during operation below minus 30°C.
Disadvantages of tin plating:
- Low wear resistance;
- Lower ductility and anti-friction properties compared to tin-lead;
- The presence of bismuth in the composition does not allow the coating to be used for food purposes;
- The coating is unstable in an alkaline environment at high temperatures, in concentrated sulfuric and hydrochloric acid.

Read also articles
Mechanism of tin plating (tinning)
What are tin and bismuth? Tin plating mechanism and coating structure

Do you want to become our client?
Just leave your request by filling out the form on the right and we will contact you as soon as possible. Thank you!

By submitting an application, you agree to processing of your personal data. Your data is protected.






















